
 |
Freethought & Rationalism ArchiveThe archives are read only. |
|
|
#81 |
|
Veteran Member
Join Date: Feb 2002
Location: Tokyo
Posts: 1,126
|
What are you ranting about now?
(Yawn...) |
|
|
|
|
#82 | |
|
Veteran Member
Join Date: Oct 2002
Location: Texas
Posts: 1,247
|
Quote:
called a neutrino. In QM, it doesn't crash into the nucleus because of its wave function. It also doesn't collide with other electrons because of the natural repulsion of like-charged particles (electrostatic force, due to the exchange of virtual photons). The energy comes from those virtual massless particles. They are force-carrying particles and being such, do not have to obey Pauli's exclusion principle which means there is no limit to the number that can be exchanged between electrons, and so they give rise to a strong force. They can also have a spin of 0 or 2. Quarks can also emit them. |
|
|
|
|
|
#83 | ||
|
Veteran Member
Join Date: Mar 2002
Location: South Dakota
Posts: 2,214
|
Quote:
Quote:
And btw, the moon IS falling. |
||
|
|
|
|
#84 |
|
Veteran Member
Join Date: Aug 2002
Location: In the land of two boys and no sleep.
Posts: 9,890
|
Kimpatsu and yguy,
Please cool the tone of your discussions. Posts with nothing more than personal critiques and flippant remarks will be deleted henceforth. Thanks in advance, Wyz_sub10, S&S Moderator |
|
|
|
|
#85 | |
|
Veteran Member
Join Date: Oct 2002
Location: Texas
Posts: 1,247
|
Quote:
|
|
|
|
|
|
#86 |
|
Veteran Member
Join Date: Jun 2002
Location: A Shadowy Planet
Posts: 7,585
|
And funny enough:
In the ground state of a hydrogen atom, the electron does have some probability of residing inside the proton! 
|
|
|
|
|
#87 | ||
|
Veteran Member
Join Date: Apr 2003
Posts: 2,199
|
Quote:
Quote:
|
||
|
|
|
|
#88 | |
|
Veteran Member
Join Date: Mar 2002
Location: South Dakota
Posts: 2,214
|
Quote:
|
|
|
|
|
|
#89 | |
|
Veteran Member
Join Date: Apr 2003
Posts: 2,199
|
Quote:
As for the QM based answers to this, they would make more sense to me at least in the sense that the electron IS the energy in this model, if I understand correctly - but that gives rise to the question of whether anyone knows what an electron is, since it seems manifest itself differently under direct observation. From what I've seen, the concept of QM is about as well understood by scientists as God is by theologians. 
|
|
|
|
|
|
#90 |
|
Moderator - Science Discussions
Join Date: Feb 2001
Location: Providence, RI, USA
Posts: 9,908
|
yguy:
Essentially this amounts to saying that an electron colliding with the nucleus is against the rules. It doesn't address the source of the electron's kinetic energy. As for the QM based answers to this, they would make more sense to me at least in the sense that the electron IS the energy in this model, if I understand correctly - but that gives rise to the question of whether anyone knows what an electron is, since it seems manifest itself differently under direct observation. What "source of kinetic energy" are you talking about here? The reason electrons violate classical expectations is not that they have enough energy to avoid falling into the nucleus, it's that the energy levels are quantized. Even in classical physics the fact that the electron is attracted to the proton would not cause it to immediately crash into the nucleus, if it had enough angular momentum it could orbit the nucleus just like earth orbits the sun. The problem is that as it travels a curved path, it's accelerating, and therefore according to the rules of classical electromagnetism it should be radiating away energy as it does so, which would cause the orbit to decay so it would spiral into the nucleus (see this page on early classical models of the atom and how they were replaced by quantum models). But quantum mechanics says that its energy emissions and angular momentum are both quantized, so it can't change orbits continuously like this (although by absorbing or emitting a quanta of energy in the form of a photon, an electron can move from one orbit to another). This isn't just an arbitrary postulate of quantum mechanics' "rules" either, it can actually be derived from the basic wave mechanics at the heart of QM. In my intro quantum physics class we calculated the wavefunction for a "particle in a box", ie a particle moving in one dimension confined to a potential well--you find that in such a situation, the particle will always have a wavelength such that a whole number of waves fit in the box, basically because wavelengths that don't have this property will interfere with themselves and cancel out. This quantization of wavelengths leads to a quantization of allowable energy levels. The derivation of this is discussed here: http://hyperphysics.phy-astr.gsu.edu...ntum/pbox.html Illustration: 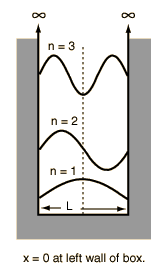 Anyway, the nucleus also creates a somewhat more complicated potential well for the electron, and in a similar way you can prove that only orbits made up of a whole number of wavelengths are allowed, and hence both orbital distance and energy are quantized in the neighborhood of the nucleus. Louis De Broglie showed that this could explain the discrete orbits in 1924, and he won a nobel prize for this work in 1929. Here's a page which talks a bit about deriving the properties of electron orbits from wave mechanics: http://www.inetarena.com/~pdx4d/snelson/Portrait4.html With a nice illustration showing how in De Broglie's model, each orbit corresponds to a whole number of wavelengths:  yguy: From what I've seen, the concept of QM is about as well understood by scientists as God is by theologians. The behavior of the wavefunction of a quantum particle--how it acts in different potential wells, how it evolves over time, etc.--is completely deterministic and is just as well-understood as the behavior of electromagnetic waves in classical physics. The only thing that makes QM more mysterious than classical theories involving waves is the "probability interpretation" of the wavefunction which says that when you make an observation, you have to take the amplitude of the wavefunction at each possible position and then square it, and that tells you the probability that the electron will be found in each possible position. But you don't really need to worry about this issue when showing why the electron has quantized orbits--that can be derived solely by looking at the wavefunction itself, and how it will behave in the potential well created by the nucleus. |
|
|
| Thread Tools | Search this Thread |
|